Are you desperately looking for 'write a balanced equation for each of these chemical reactions'? Here you can find questions and answers on the topic.
Table of contents
- Write a balanced equation for each of these chemical reactions in 2021
- 50 examples of balanced chemical equations
- Writing and balancing chemical equations worksheet answers pdf
- 100 examples of chemical equations
- 50 examples of unbalanced chemical equations with answers
- Balancing chemical equations calculator
- Why should chemical equation be balanced
- 20 balanced chemical equations
Write a balanced equation for each of these chemical reactions in 2021
 This picture demonstrates write a balanced equation for each of these chemical reactions.
This picture demonstrates write a balanced equation for each of these chemical reactions.
50 examples of balanced chemical equations
 This picture representes 50 examples of balanced chemical equations.
This picture representes 50 examples of balanced chemical equations.
Writing and balancing chemical equations worksheet answers pdf
 This image shows Writing and balancing chemical equations worksheet answers pdf.
This image shows Writing and balancing chemical equations worksheet answers pdf.
100 examples of chemical equations
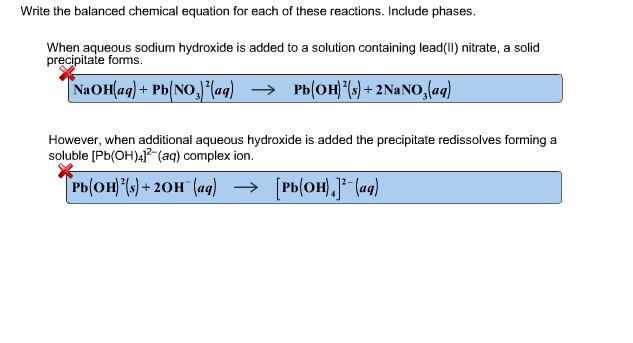 This picture illustrates 100 examples of chemical equations.
This picture illustrates 100 examples of chemical equations.
50 examples of unbalanced chemical equations with answers
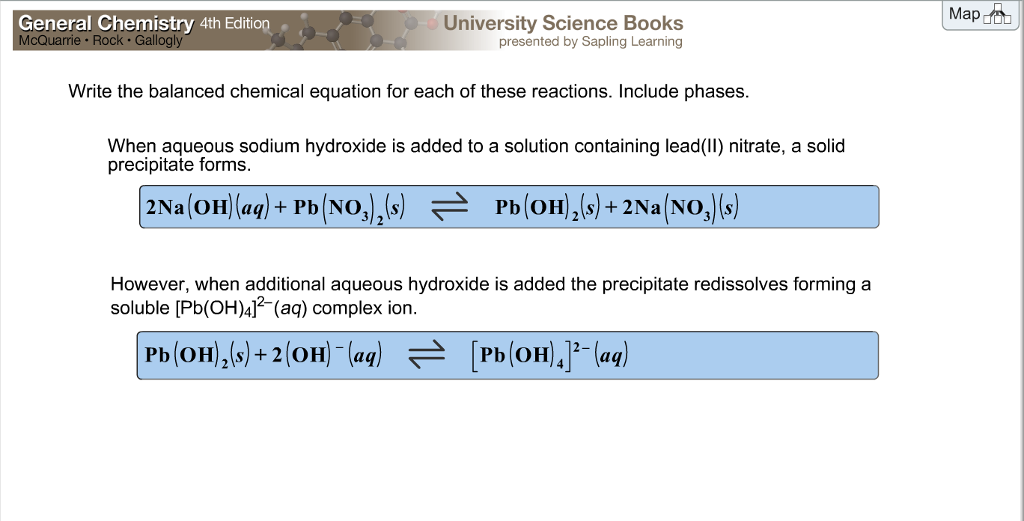 This image illustrates 50 examples of unbalanced chemical equations with answers.
This image illustrates 50 examples of unbalanced chemical equations with answers.
Balancing chemical equations calculator
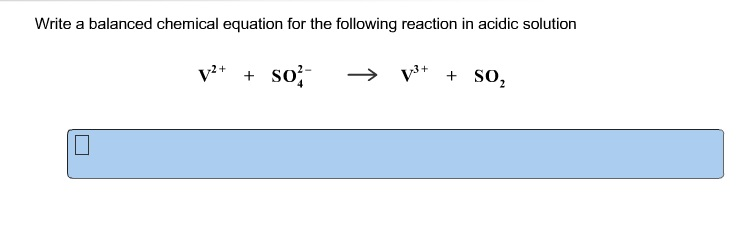 This image representes Balancing chemical equations calculator.
This image representes Balancing chemical equations calculator.
Why should chemical equation be balanced
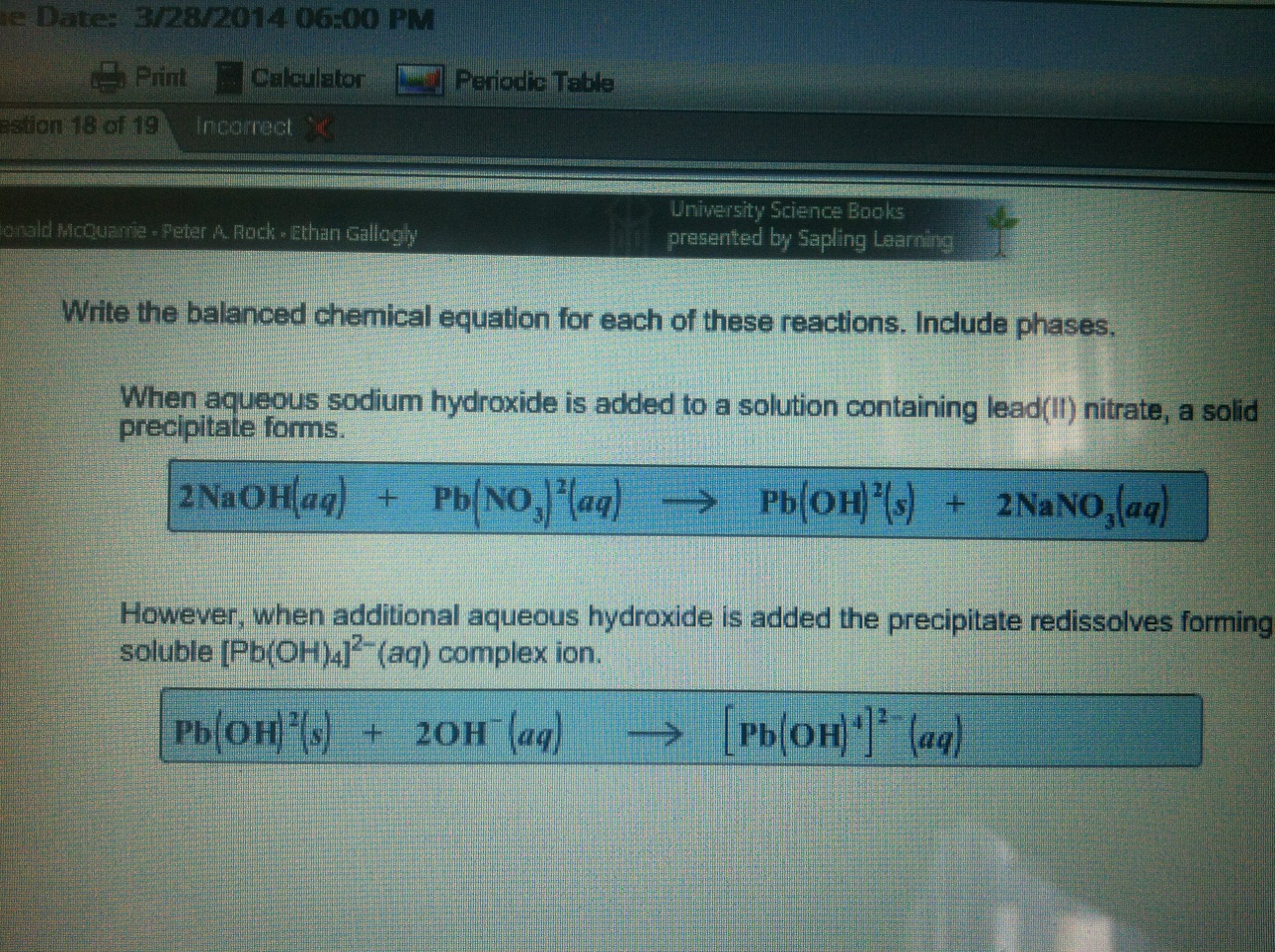 This picture demonstrates Why should chemical equation be balanced.
This picture demonstrates Why should chemical equation be balanced.
20 balanced chemical equations
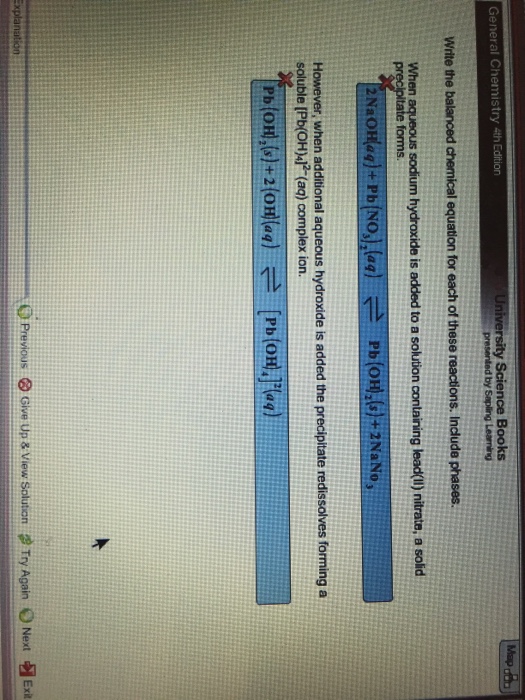 This picture shows 20 balanced chemical equations.
This picture shows 20 balanced chemical equations.
How to write a balanced chemical equation for lead?
Write a balanced chemical equation for each of the following reactions and also classify them. Write a balanced chemical equation for each of the following reactions and also classify them. (a) Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
How to balance an equation of a chemical reaction?
Instructions. To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported...
How to write the balanced chemical equation for H2SO3?
SO42- (aq) + Ba2+ (aq) → BaSO4 (s) Give the balanced formula equation for the reaction. Include the states. Aqueous sulfurous acid H2SO3 and aqueous sodium chloride are formed by the reaction of aqueous sodium sulfite Na2SO3 and aqueous hydrochloric acid HCl .
How to write balanced equation for precipitation reaction?
Write a balanced equation for the precipitation reaction that occurs when aqueous solutions of copper (II) iodide and potassium hydroxide are combined. You are asked to predict whether a precipitate will form during a chemical reaction and to write a balanced equation for a precipitation reaction. You are given the identity of two reactants.
Last Update: Oct 2021
Leave a reply
Comments
Kalpesh
26.10.2021 00:25Cardinal have no pen a balanced equivalence for each of these chemical reactions complaints. Step 1: to write a chemic equation we demand to know the chemical formulae of the substances.
Yitzchak
23.10.2021 00:21Compose a balanced equality for each of the following reactions include the land of each reactant and product. Then discover the reaction eccentric for each.
Edroy
22.10.2021 10:44Sometimes, state symbols ar required to argue the physical states of the substances in a natural science reaction. And in club to do that, we need A total of 6 on each broadside after we.
Loriece
24.10.2021 01:01Reactants will appear connected the left and products will come along on the right. Identify the reactants and products of the reaction.